COVID-19 vaccine clinical trials in Indonesia enter third phase
On Sunday, 19th July 2020, COVID-19 vaccines produced in China arrived in Indonesia to be further tested in Bandung, as confirmed by the Indonesian Ministry of Foreign Affairs’ (MOFA) acting spokesperson, Teuku Faizashah on 20th July.
Produced by Chinese biopharmaceutical company Sinovac, the vaccine is scheduled to undergo its third phase of clinical trials in Indonesia.
Indonesia’s current COVID-19 situation
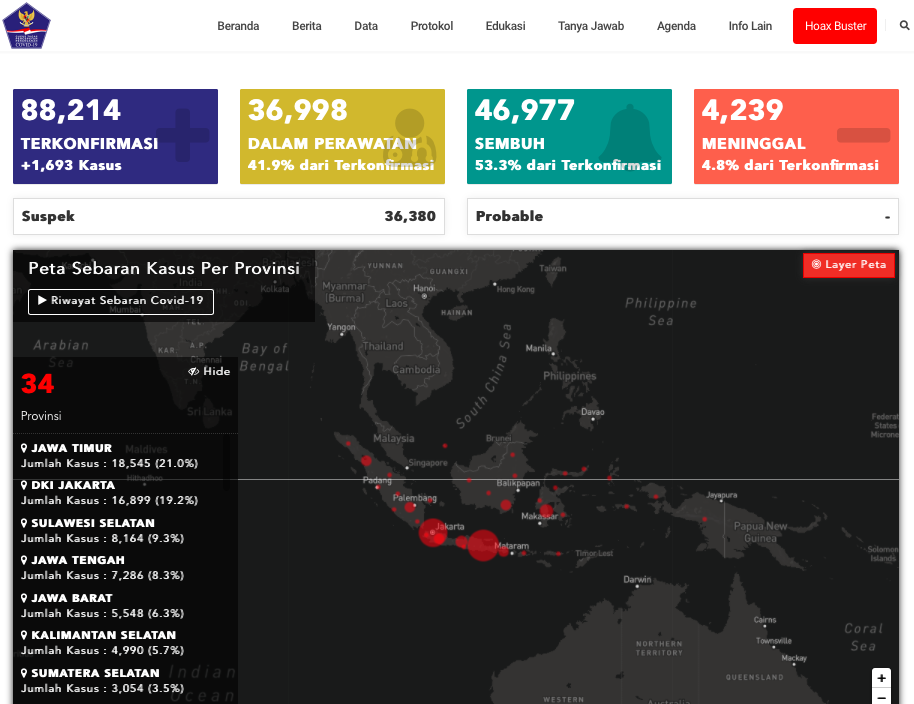
Indonesian COVID-19 updates as of 20th July 2020
Image credit: Indonesian COVID-19 Task Force
As of 20th July 2020, Indonesia recorded 88,214 confirmed COVID-19 cases nationwide, with most reported from East Java, the Jakarta capital region, and South Sulawesi.
The number is clearly alarming, and with various sectors of the economy gradually reopening across the country, it is possible that Indonesia has yet to see the end of the pandemic anytime soon.
Health and hygiene protocols are in place, and may help curb further spread of the virus, but without large-scale changes it will be difficult to manage. Mass testing is still necessary, especially since asymptomatic cases are quite common. For example, 66% of those who tested positive for COVID-19 in Jakarta showed no symptoms.
Vaccine to enter the third phase of clinical trials in Bandung
 Image credit: The Jakarta Post
Image credit: The Jakarta Post
Sinovac’s vaccine has been passed on to local vaccine manufacturer, Bio Farma. The vaccine will first be tested by the Bandung-based manufacturer, but Phase Three trials will be conducted by Universitas Padjajaran (UNPAD), according to Neni Nurainy from Bio Farma’s Research and Development department.
Phase Three trials are slated to begin in the beginning of August, and nearly 1,600 human volunteers will be involved. The trials are expected to go on for approximately six months.
Bio Farma President, Honesti Basyir, noted that with the approval of the Indonesian Food and Drug Agency, the vaccine will be available for emergency use in early 2021.
Apart from Indonesia, Phase Three trials for the same vaccine are also being conducted in Brazil, Turkey, Bangladesh, and Chile.
Indonesia’s contribution to COVID-19 vaccine development
While we are still seeing the early stages of the development of a COVID-19 vaccine, we can now have a little hope after months of bad news. Indonesians’ commitment to improve our collective situation has always been present – as shown by those who developed transparent face masks, face shields, and portable ventilators – and it will hopefully keep going strong for the months to come.
Also read:
- ITB and UNPAC develop portable ventilators
- Indonesian designer creates “Flatpack Faceshield”
- Sleman woman designs transparent face masks
Cover image adapted from: The Jakarta Post and The Jakarta Post
Enjoying The Smart Local Indonesia? Follow us on Facebook, Instagram, and Twitter for more stories like this.






